Power module execution is extremely delicate to the stream pace of the reactants, so it is essential that every cell in a stack gets roughly similar measure of the reactants. Each cell active area in a stack must receive a uniform amount of reactant gases because the performance of a fuel cell is dependent on the flow rate of the reactants. The flow field can be square, rectangular, circular, hexagonal, octagonal, or irregular, with square and rectangular being the most common shapes. The orientation of the flow field can be either horizontal or vertical. The direction of the stream field might affect the fluid water evacuation during the closure of the stack. A serpentine channel flow field has been demonstrated by numerous researchers to be an excellent option for HT-PEMFC stacks.
The size of the ribs and channels has a big impact on how well a fuel cell works. The dimensioning effects on cell performance can occur in a variety of ways:
•pressure level and differential tension;
• removing the condensate;
• conductivity of electricity;
• media transport characteristics
The differential strain impacts both the condensate evacuation properties and the tension level fundamentally in the cell gulf area, hence affecting energy as well as cell water family by changes in RH. Additionally, differential pressure’s impact on auxiliary energy demand must be taken into account.
Performance results
Empirical research using membranes made by increasing local acid (proton carrier) concentrations and learning about the structure–property advantages of various polymer architectures has led to improved fuel cell performance. The amount of acid in the membrane is increased until either the solubility in boiling water or in situ fuel cell performance can be optimized to determine an upper limit of IEC.
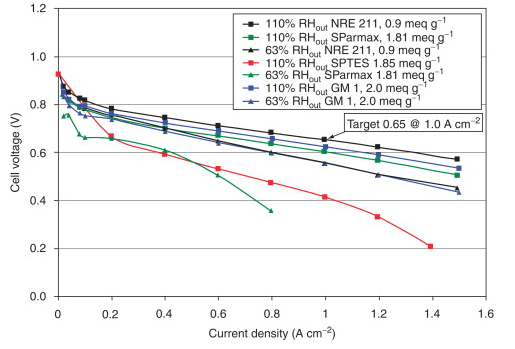
Our in situ tests show that proton-exchange membranes with hydrophilic volume IECs between 2.0 and 3.4 meqH+ perform well. These aromatic hydrocarbon membranes have an ex situ in-plane proton conductivity of 1.85 meq g-1, which is comparable to that of SPTES but lower than that of Nafion NRE-211 at 50% relative humidity. However, the ex situ in-plane proton conductivity does not always correlate well with the performance of an in situ fuel cell, as was previously mentioned. The in situ fuel cell performance of these new membranes is comparable to that of NRE-211 at 110% RHout, surpassing that of any other aromatic hydrocarbon-based membrane we have prepared and tested. Figure 9 displays the outcomes. Even at low RH, improved performance has been demonstrated; in a fuel cell with an RHout of 63% and an inlet RH of 32%, GM 1 PEM performs similarly to NRE-211.
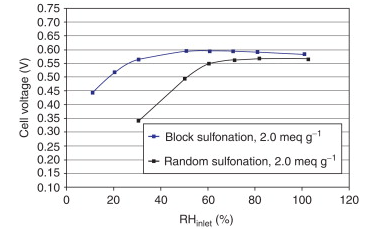
Block polymer structures extraordinarily upgrade the collection of the sulfonic corrosive practical gatherings, hence working with better execution at lower RH. Figure 10 shows the exhibition aversion to channel RH at 1.2 Acm−2 and 80 °C of block and arbitrary polymers. The (sulfonated) block layer exhibits around a 50-mV better execution at half gulf RH and a great 0.565 V at 30% channel RH contrasted with 0.342 V for the irregular film under similar circumstances. The two films have a similar thickness and IEC. The block structure helps to reduce the membranes’ reliance on water to maintain the percolation threshold required for proton transport and boosts the local acid concentration of sulfonic acid groups. In haphazardly sulfonated polyaromatic layers, with moderately solid polymer chains, the corrosive destinations have a restricted capacity to total and mass free water is compelled to go about as the course for protons over the enormous distances between charge transporters. When compared to PFSA, SPEEK has a smaller hydrophilic domain and a random distribution of sulfonic acid groups, making it more dependent on water to maintain the percolation pathway necessary for vehicular or Grotthus hopping proton transport. Because the charge carriers are closer together, the improved morphology makes block polymers with the same IEC and chemistry less dependent on bulk water. By adjusting the length of the hydrophilic and hydrophobic blocks as well as the degree of sulfonation in the hydrophilic block, a structure–property relationship can be changed to improve fuel cell performance.