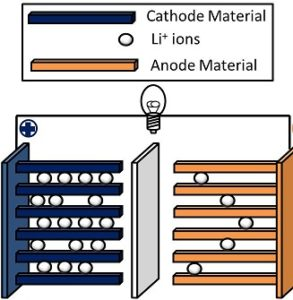
An advanced battery technology known as a lithium-ion battery makes use of lithium ions as a crucial component of its electrochemistry. Lithium atoms in the anode are ionized and separated from their electrons during a discharge cycle. From the anode, the lithium ions move through the electrolyte until they reach the cathode, where they reunite with their electrons and neutralize the electrical charge. Between the anode and cathode, a micro-permeable separator allows the lithium ions to move through. Li-ion batteries are able to have a very high voltage as well as charge storage per unit mass and volume in part due to lithium’s small size—it ranks third only to hydrogen and helium.
What is a lithium-ion battery used for?
Electronics, toys, wireless headphones, handheld power tools, small and big appliances, electric cars, and electrical energy storage systems are just a few of the devices that employ lithium-ion (Li-ion) batteries.
What are the 3 types of lithium batteries?
- There are three types of cells that are used in lithium batteries: cylindrical, prismatic, and pouch cells.
What are the use of lithium-ion battery?
Lithium-ion batteries are commonly used in a variety of electronic devices, including:
- Portable electronics: Lithium-ion batteries power a wide range of portable electronic devices, including smartphones, laptops, tablets, and cameras.
- Electric vehicles: Lithium-ion batteries are used to power electric vehicles, such as electric cars and electric bicycles, due to their high energy density and ability to provide long-range driving.
- Renewable energy storage: Lithium-ion batteries can be used to store energy generated from renewable sources, such as solar and wind power. This helps to balance power supply and demand and improve the efficiency of renewable energy systems.
- Power tools: Lithium-ion batteries are commonly used in power tools, such as drills and saws, due to their high energy density and lightweight design.
- Medical devices: Lithium-ion batteries power a variety of medical devices, including pacemakers and defibrillators, due to their reliability and long lifespan.
Overall, lithium-ion batteries offer a high power density, low self-discharge rate, and long cycle life, making them an ideal choice for a wide range of applications.
As electrodes, Li-ion batteries can make use of a variety of materials. The combination of graphite (anode) and lithium cobalt oxide (cathode), which is most frequently found in portable electronic devices like cellphones and laptops, is the most common. Lithium iron phosphate and lithium manganese oxide, both of which are utilized in electric and hybrid automobiles, are additional cathode materials. The electrolyte of most Li-ion batteries is typically ether, a class of organic compounds.
What is the biggest problem with lithium batteries?
Lithium-ion batteries contain metals such as cobalt, nickel, and manganese, which are toxic and can contaminate water supplies and ecosystems if they leach out of landfills. Additionally, fires in landfills or battery-recycling facilities have been attributed to inappropriate disposal of lithium-ion batteries.
What is the raw material of lithium-ion battery?
Critical raw materials used in manufacturing Li-ion batteries (LIBs) include lithium, graphite, cobalt, and manganese. As electric vehicle deployments increase, LIB cell production for vehicles is becoming an increasingly important source of demand.
Which lithium battery is best?
LiFePO4 is now known as the safest, most stable and most reliable lithium battery.

Environmental impacts of lithium-ion batteries –
Lithium-ion batteries are commonly used in a variety of electronic devices, including electric vehicles and portable electronics. However, the production and disposal of lithium-ion batteries have several environmental impacts, including:
- Extraction of raw materials: The production of lithium-ion batteries requires the extraction of raw materials such as lithium, cobalt, and nickel, which can have negative environmental impacts. Mining activities can cause soil erosion, water pollution, and habitat destruction.
- Energy consumption: The production of lithium-ion batteries requires a significant amount of energy, which can lead to increased greenhouse gas emissions and contribute to climate change.
- Water consumption: The production of lithium-ion batteries requires large amounts of water, which can contribute to water scarcity and put stress on local water resources.
- Pollution from manufacturing: The production of lithium-ion batteries can release pollutants such as sulfur dioxide, nitrogen oxides, and particulate matter, which can contribute to air pollution.
- Disposal: Lithium-ion batteries can contain toxic materials such as lead, cadmium, and mercury, which can leach into the soil and water if not disposed of properly. Improper disposal can also lead to the release of greenhouse gases, which can contribute to climate change.
It is important to consider the environmental impacts of lithium-ion batteries and to work towards developing more sustainable and environmentally friendly battery technologies.
What are the disadvantage of lithium-ion battery
While lithium-ion batteries offer several advantages, they also have some disadvantages, including:
- Limited lifespan: Lithium-ion battery has a limited lifespan and eventually lose their ability to hold a charge. This can result in reduced battery life and performance over time.
- High cost: Lithium-ion batteries are more expensive than other types of batteries due to the cost of the raw materials and production process.
- Risk of thermal runaway: If not designed and manufactured properly, lithium-ion batteries can experience thermal runaway, a process that causes the battery to overheat and potentially catch fire or explode.
- Sensitive to temperature: Lithium-ion batteries can be sensitive to extreme temperatures. Exposure to high temperatures can cause damage to the battery, while exposure to low temperatures can reduce battery performance.
- Environmental concerns: The production and disposal of lithium-ion batteries can have negative environmental impacts, as mentioned in the previous answer.
It is important to consider these disadvantages when choosing a battery for a specific application and work towards developing more sustainable and environmentally friendly battery technologies.
References – Lithium-ion battery