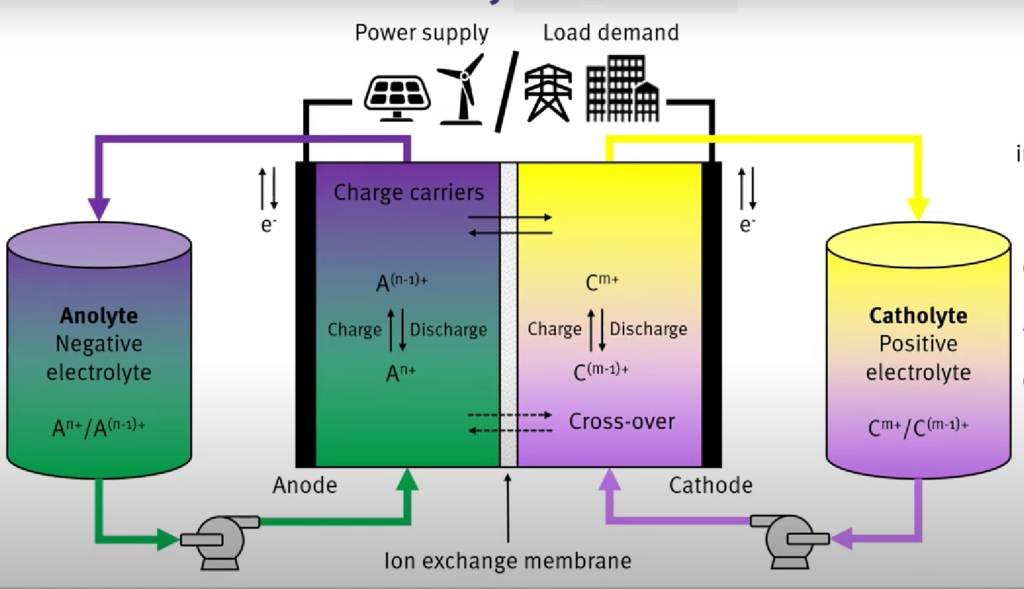
What is a redox flow battery?
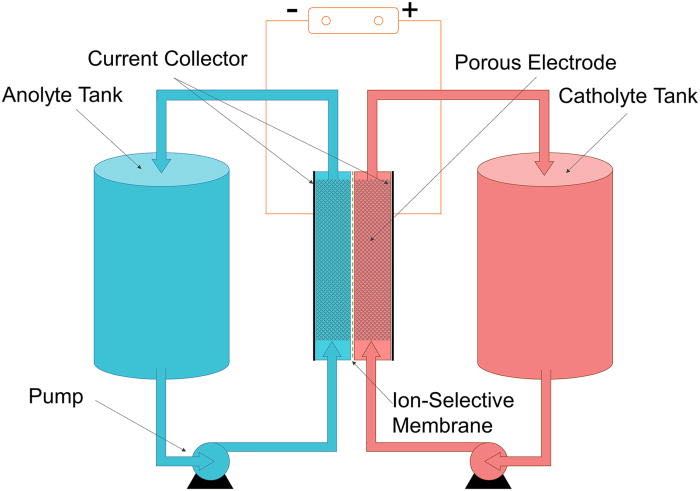
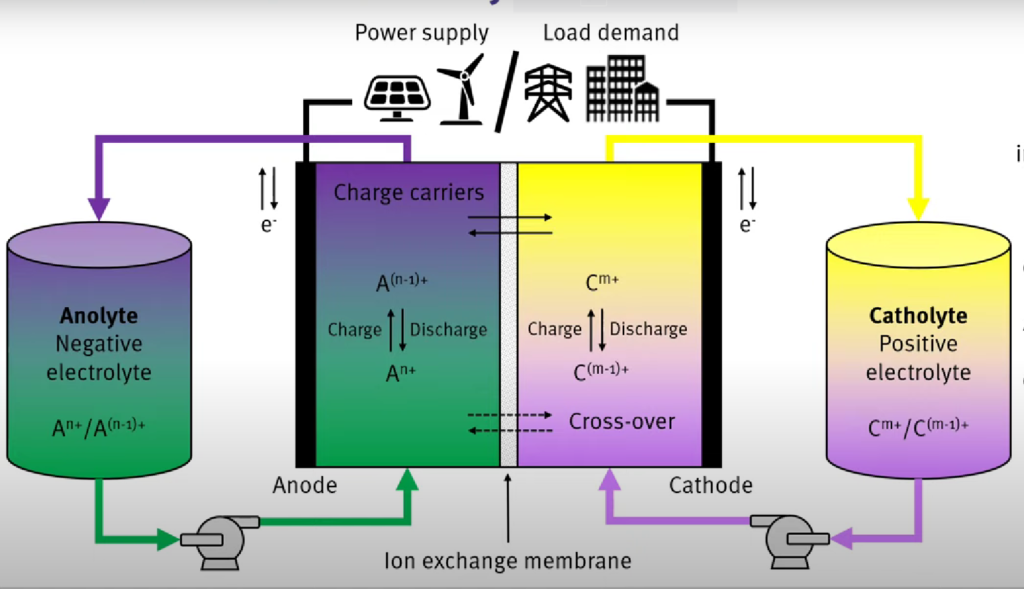
Redox flow batteries (RFB) represent one class of electrochemical energy storage devices. The name “redox” refers to chemical reduction and oxidation reactions employed in the RFB to store energy in liquid electrolyte solutions which flow through a battery of electrochemical cells during charge and discharge.
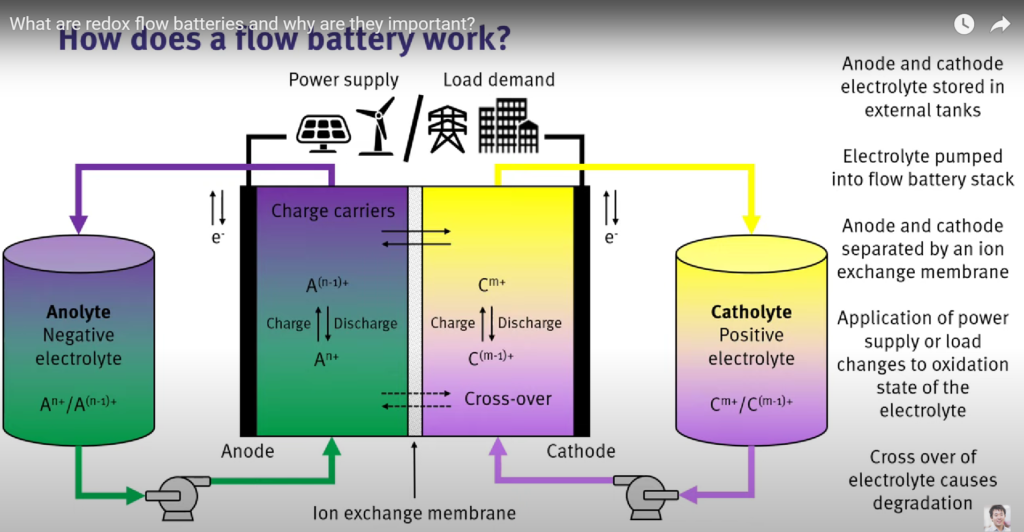
During discharge, an electron is released via an oxidation reaction from a high chemical potential state on the negative or anode side of the battery. The electron moves through an external circuit to do useful work. Finally, the electron is accepted via a reduction reaction at a lower chemical potential state on the positive or cathode side of the battery. The direction of the current and the chemical reactions are reversed during charging.
How does a redox flow battery work?
A redox flow battery is an electrochemical energy storage device that works on the basis of converting chemical energy into electrical energy through reversible oxidation and reduction of working fluids.
Comparison between Redox Flow Battery & Lithium Ion Battery?
Key differences between flow batteries and lithium ion ones include cost, longevity, power density, safety and space efficiency. if your main priority when it comes to battery energy storage systems is either longevity or safety, flow batteries are likely the ideal technology for you. If your main energy storage system priority is either cost or space efficiency lithium ion batteries are likely the idea technology for you. If power density is your main concern, either technology could suit your unique needs, depending on the length of battery cycle you require.
Types and configurations of redox flow batteries.
Conventional redox flow batteries have two divided electrolyte reservoirs (Figure 2a). Catholyte and anolyte are separated by a membrane, which permits ions to pass through it. The most common working ions in aqueous media, H+ (349.8 S cm2 mol−1) and OH− (198.0 S cm2 mol−1), have the highest limiting molar conductivity among all known cations and anions, respectively [4]. All‐vanadium redox flow batteries, for instance, have V3+/V2+ redox reactions on the negative side (anolyte) and VO2+/VO2+ on the positive side (catholyte). Such a battery uses the same metal ions on both sides. Crossover of metal ions through the membrane will then not cause contamination of the electrolyte. In contrast, for redox flow batteries with different metal ions such as Fe3+/Fe2+ and Cr3+/Cr2+ in an iron‐chromium flow battery, the cross‐contamination via ion penetration may cause irreversible performance loss.
Figure 2.
Configurations of (a) a conventional redox flow battery with two divided compartments containing dissolved active species, (b) a hybrid redox flow battery with gas supply at one electrode, (c) a redox flow battery with membrane‐less structure and (d) a redox flow battery with solid particle suspension as flowing media.
What are the disadvantages of redox flow batteries?
Their disadvantages are low energy density and low charge/discharge rates. Flow batteries can be found in capacities ranging from 25 kW to more than 100 MW. Flow batteries are heavier than lithium ion batteries and they also take up more space due to their considerably sized tanks. In comparison, lithium ion batteries are more portable and won’t take up as much of your space.
What is the key advantage of a redox flow battery?
Redox flow batteries offer an economical, low vulnerability means to store electrical energy at grid scale. Redox flow batteries also offer greater flexibility to independently tailor power rating and energy rating for a given application than other electrochemical means for storing electrical energy.
How long do redox flow batteries last?
Redox Flow Batteries lasts up to 20 Years.
| Storage technology | Power capacity/density | Lifetime |
| Lead-acid battery | 30–50 Wh/kg | 3–15 years or 500–1000 cycles |
| Sodium-sulfur battery | 150–250 Wh/kg | 10–15 years or 2500–40000 cycles |
| Lithium-ion battery | 200 Wh/kg | 10–15 years or 3000 cycles |
| Vanadium redox flow battery | 16–33 kWh/m3 | 5–20 years or 1500–15000 cycles* |
How long do redox flow batteries last?
Flow batteries have almost an unlimited battery cycle life because of the absence of phase-to-phase chemical reactions. This technology can by cycled every day for up to 30 years.
How efficient are redox flow batteries?
The Coulombic efficiency (ratio of the discharge capacity/charge capacity) of this representative desalination/battery cycle was 99.3%, indicating that most of the charge stored in each redox couple during desalination was regenerated during battery discharge
Do flow batteries have a future?
They use rare non-renewable materials, and they cannot scale up to meet the imperative for universal green energy. Forward thinkers believe flow batteries are our future alternative, with potential to carry us forward.
How much does a redox flow battery cost?
The cost of materials used in redox flow battery alone is about 1/30th that of the materials needed for a li-ion, which tends to use expensive elements like cobalt. New Redox Flow Battery Design Will Cost $25 Per kWh Or Less
Are redox flow batteries rechargeable?
Redox flow batteries are rechargeable batteries that are charged and discharged by means of the oxidation-reduction reaction of ions of vanadium.
Conclusions and perspectives
Redox flow battery technology is relatively new and not yet well‐developed. Rational electrolyte management and cell design can lead to the enhancement of energy storage capability and a reduction in construction cost. Novel electrolyte chemistry and development of a new configuration of flow batteries will create high system flexibility. Physiochemical and electrochemical redox properties of active couples, stability window of supporting electrolyte, selection of supporting ions, stability of electrode materials and cell components are key factors for successful applications. Future market penetration of flow batteries needs low cost, high energy density and high power density. The pace of recent development in the active organic molecules as electrolytes opens new strategies of cost‐effective and sustainable solutions for large‐scale stationary energy storage. The application of energy‐dense solid materials in suspension for redox flow batteries may largely enhance the energy density of flow battery systems.
[ays_quiz id=”5″]